According to relevant data, China uses approximately 7% of global freshwater, 8% of arable land, and 12% of grasslands to raise 50% of the world's pigs, 10% of cattle, 20% of sheep, 30% of chickens, and 80% of freshwater organisms. The density of animal farming in China is about 30 large animal units per square kilometer, which is 2.4 times the global average breeding density. The problems of high breeding density, high breeding costs, and heavy animal epidemic prevention and control tasks will persist for a long time.

Veterinary medicine and veterinary biological products are both sub sectors of the animal protection industry. Among them, veterinary chemicals and veterinary traditional Chinese medicine: including raw materials and various preparations synthesized from raw materials, including antimicrobial drugs, antiparasitic drugs, etc; Veterinary biological products: including preventive biological products (veterinary vaccines, used for immunization), therapeutic biological products, diagnostic biological products, etc. Among them, veterinary vaccines are absolutely dominant, accounting for about 80%. In comparison, the development space of veterinary chemical drugs and veterinary traditional Chinese medicine is limited, and veterinary biological products are more in line with the development direction of animal health.
Veterinary vaccines are the largest component of veterinary biological products in China and the main means of preventing and treating infectious diseases in animals. From the perspective of segmented fields, the industry presents three major characteristics: (1) the proportion of domestic pig and avian vaccines is relatively high, and the pet vaccine market is still in its infancy; (2) The market share of pig and avian vaccines is relatively high, while foot-and-mouth disease and avian influenza account for half of the total sales of veterinary vaccines; (3) Benefiting from the policy of "hitting first and then supplementing", the market share of non mandatory immunization vaccines has steadily increased in recent years. The profit margin of products with superimposed veterinary biological products is much higher than that of products such as veterinary chemicals and important veterinary drugs. Products such as veterinary vaccines are expected to further develop.
When it comes to veterinary vaccines, it may be inevitable for everyone to make a certain comparison with human vaccines. What is the difference between veterinary vaccines and human vaccines? The editor hereby quotes the viewpoint of Dr. Ma Ningning, the founder of Dingzhi Biology: Human biological products and animal biological products are essentially interconnected, so animal biological products are a part of the research and development and production of many overseas pharmaceutical companies. The largest animal protection companies in the world are the animal protection departments or subsidiaries of pharmaceutical companies such as Pfizer, Bringer, MSD, and Lilly. In China, animal biological products are a relatively independent field, which leads to the improvement of product quality and R&D week for animal biological products both domestically and internationally There is a certain gap in terms of time and other aspects, which has created a great space for development.
In recent years, with the continuous deepening of China's development in the field of biological products, the research and production of veterinary biological products have also made significant progress. The process of developing veterinary vaccines can generally be divided into two stages: upstream and downstream. Upstream production mainly involves the use of microbial technology to develop target products, while downstream production mainly involves further processing, concentration, and purification of the target product solution to ensure that the produced veterinary vaccines meet national standards. However, in actual industrial production, the laboratory stage and pilot scale production stage still cannot be effectively connected, hindering the further large-scale production of veterinary vaccines; Secondly, the antigen separation and purification technology of downstream veterinary vaccines needs to be further strengthened to ensure the quality and immune efficacy of veterinary vaccines.

Veterinary vaccines are usually a specific immune protein, and there are many differences in protein composition, physicochemical properties, biological properties, and other aspects among different veterinary vaccines. Therefore, they also face many challenges in downstream production.
At present, the methods commonly used in the production of animal vaccines in China are the first and second generation virus amplification systems. However, there are also many problems, such as ineffective improvement in production efficiency, high production costs, high content of other proteins in the target substance, and frequent occurrence of immune side reactions.
Production efficiency issue: mainly due to the fact that the first generation of virus amplification methods were mostly in the mode of rotary flask culture, where cells adhered to the wall and grew inside the rotary flask, and manual operations were mostly used, resulting in no improvement in production capacity and efficiency.
Production cost issue: Although the second generation virus amplification system has some improvement in production capacity compared to the first generation, it requires high concentration of serum, high production costs, and overall stability deviation of the product. In addition, the high content of other proteins in the target substance cannot effectively induce immune reactions, and will produce strong immune side reactions.
The above issues are also urging us to adjust and optimize the downstream production process of veterinary vaccines, ensure the antigen content of veterinary vaccines, reduce impurity protein content, and ensure that the corresponding veterinary biological products meet the requirements of modern animal epidemic prevention.
At present, the mainstream process optimization is mainly reflected in the downstream production process, with the separation and purification stage of the target protein being the most critical. Before selecting the corresponding separation and purification method, it is necessary to conduct a comprehensive analysis of the target protein, understand the physical and chemical properties, composition, and other key information of the protein, in order to determine the corresponding separation and purification method. Therefore, we can consider and choose the appropriate process route from the following dimensions.
We all know that proteins are a class of biological macromolecules composed of multiple amino acids, and the molecular weight of proteins is closely related to the corresponding separation and purification methods. For example, ultrafiltration separation technology is used in the preparation of bivalent inactivated vaccines against foot-and-mouth disease in pigs, and this technology is also used in the preparation of pseudorabies vaccines to improve the purity of target proteins.
There are many factors affecting protein solubility, such as pH value of mixed solution, ionic strength and temperature of mixed solution. Under the same conditions, there are significant differences in the solubility of different antigen protein structures. Combining the structural differences of different antigen proteins, changing certain conditions can accelerate the separation and purification of proteins. The purification of animal vaccine antigens mainly involves isoelectric precipitation and pH regulation methods.
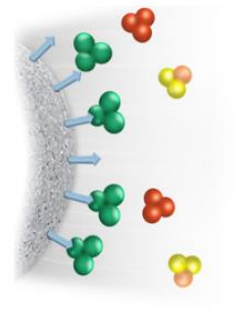
The target protein has specific characteristics and can recognize certain specific molecules, which can be used to construct corresponding biological macromolecule separation and purification methods. Affinity chromatography technology has been widely used in the production of target proteins using this principle.
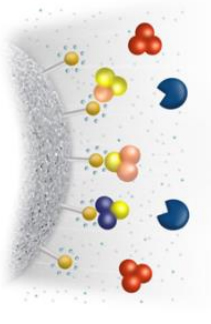
In the process of protein separation and purification, if there is a difference in adsorption between the target protein and other impurity proteins, this characteristic can be used to separate and purify the target protein. The main principle is that the hydrophobic groups coupled on the stationary phase carrier reversibly bind with the hydrophobic molecules in the mobile phase to achieve effective separation of the target protein.
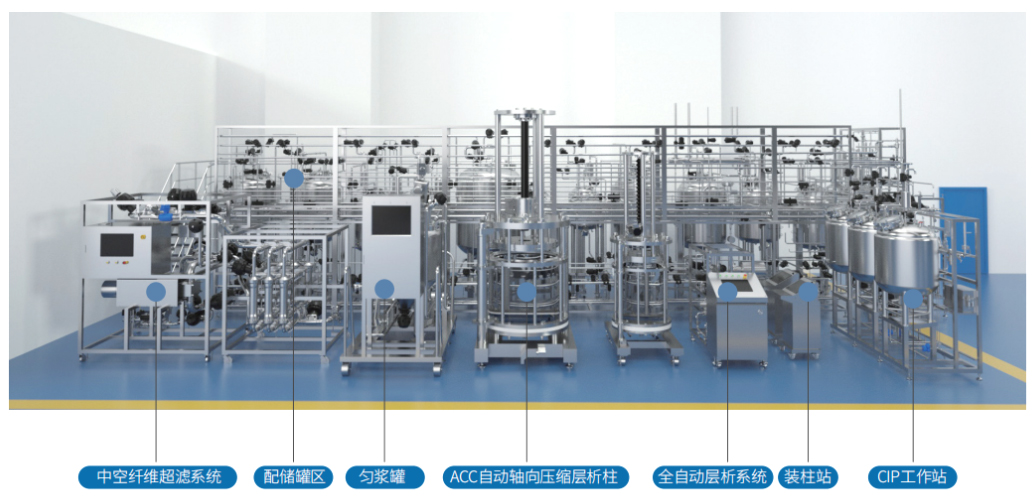
For the downstream process of veterinary vaccine production, Hanbang Technology can provide relevant customers with a comprehensive solution for downstream purification. In this process, relevant equipment such as ACC automatic axial compression chromatography column (including chromatography system), automatic tangential flow filtration system, mixing tank, mixing system, homogenization tank, online cleaning workstation, etc. will be mainly involved.
Hanbang Technology can provide Turnkey services to customers through EPC or flexible and diverse modes, including but not limited to the design of sample pre-treatment modules, chromatography modules, ultrafiltration modules, as well as the entire system automation control, public engineering and other solutions, as well as the selection, supply, installation, pipeline construction, etc. of corresponding production line equipment, and provide verification documents that meet GMP and FDA requirements.